Scientists and educators may have to fundamentally rewrite organic chemistry textbooks. Recent research by a team from Cardiff University’s School of Chemistry suggests that alkyl groups may actually pull electrons away from the rest of the molecule. This directly contradicts the century-long belief that alkyl groups donate electrons through an inductive effect.
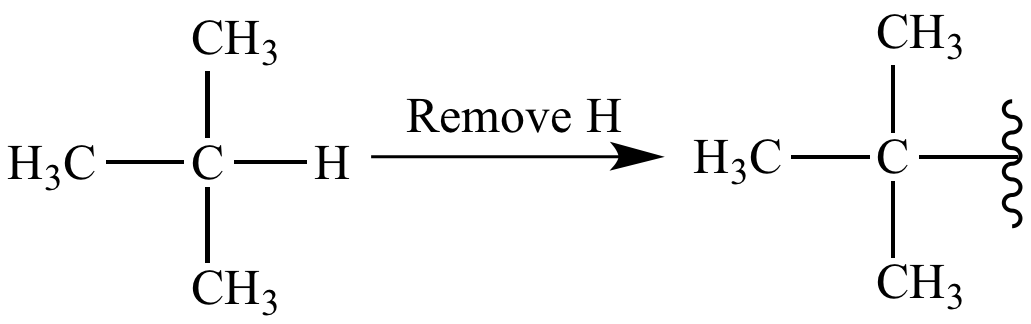
Alkyl groups are a chain of single-bonded carbon and hydrogen atoms. In comparison to the basic alkane structure, alkyl groups have one less hydrogen atom, which allows them to attach to another parent chain and create a branched molecule. Commonly known as one of the R-groups in chemistry, they can affect the chemical and physical properties of the parent chain.
According to UCLA’s Chemistry Department, an inductive effect is “the effect on electron density in one portion of a molecule due to electron-withdrawing or electron-donating groups elsewhere in the molecule.” Chemist Christopher Ingold first concluded that alkyl groups must be electron-donating in character in the 1920s to explain trends in the pH of organic molecules, specifically the acidity of carboxylic acids and the basicity of amines. Textbooks quickly adopted his theory, which students still learn about today.
However, carbon is actually more electronegative than hydrogen, meaning an alkyl group should theoretically be electron-withdrawing, pulling electrons away from the molecule. Elliot’s team used advanced computational methods to prove that alkyl groups actually exert a slight electron-withdrawing inductive effect. Overall, though, since alkyl groups evidently seem to exhibit electron-donating character, the published findings by lead author Dr. Mark Elliot conclude this effect must be from other, more dominant factors. According to Chemical and Engineering News, one such factor is hyperconjugation, the stabilizing effect from interactions between certain orbitals on neighboring atoms.
Many hope this new discovery can inspire scientists and educators to focus on accuracy rather than simplicity, especially at the secondary school level. According to organic chemist Jonathan Clayden, inductive effects are simpler to explain than molecular orbital theory. This is why secondary school teaching often explains trends with inductive effects while undergraduate teaching would explain the same trends using conjugation.
“I’m very opposed to the situation that we often find ourselves in as university educators where we have to say to our students, ‘You learned this before but it’s not true,’” Elliot said. “If I was rewriting one of my books, I would have a short statement [explaining how] you do not need to worry about inductive effects for alkyl groups because, if the electronic effect is significant, it will not be an inductive effect!”